Neuroscience and Medical Projects
Parkinson’s Disease - ENIGMA-PD
Over 10 million people worldwide live with Parkinson’s Disease (PD) - the world’s second most prevalent neurodegenerative disease (Parkinson’s Disease Foundation, 2017). There is no cure for PD, and current PD treatments variably attenuate symptom severity and ultimately do not prevent disease progression. Neuroimaging studies of PD have the potential to help identify mechanisms underlying disease progression and risk factors for disease severity or minimal treatment response, yet study findings have been varied. Wellpowered, international efforts pooling brain imaging data from diverse PD patients can help understand disease progression, identify factors that influence the disease, and potentially lead to new PD treatments by improving the efficiency of clinical trials.
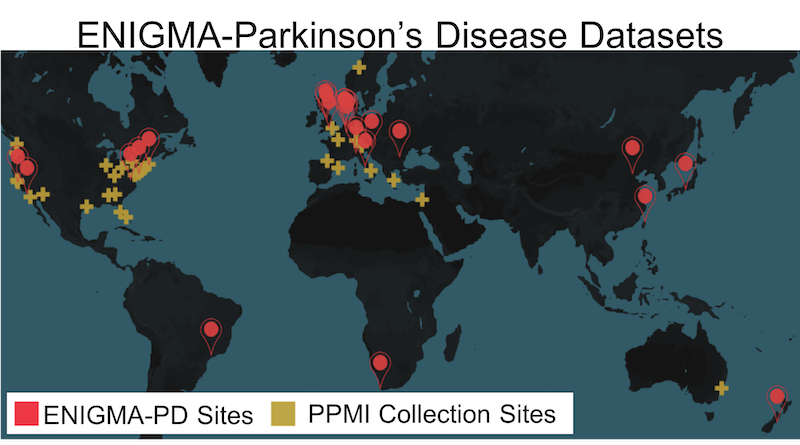
Through our ENIGMA-Parkinson’s Disease initiative, we present the largest and most highly powered neuroimaging investigation of PD by pooling data from 21 sources worldwide. Using advanced neuroimaging metrics, we aim to identify factors that impact disease severity and progression, allowing us to biotype patients into progression and treatment efficacy groups, and in turn, supporting the development of personalized treatments. Using global data to boost power, we tackle pressing questions in PD: How does the illness, symptom severity, and treatment response relate to brain structure, neural connectivity, and functional synchrony? Do genetic susceptibility loci for PD help predict brain decline? What PD subtypes, or clusters, can imaging identify?
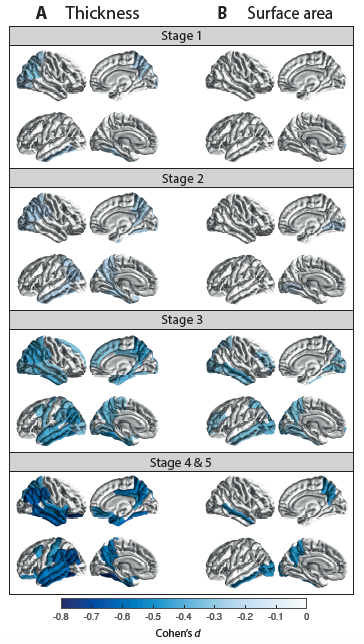
Cortical thickness and surface area group differences for PD patients vs controls. Cohen’s d effect size estimates are shown, for differences in (A) cortical thickness and (B) cortical surface area. A negative d-value indicates smaller measurements in PD patients. Cortical regions with P-values < 7·3510-4 (i.e., 0·05/68 ROIs) are depicted in the heatmap colors.
Further Reading
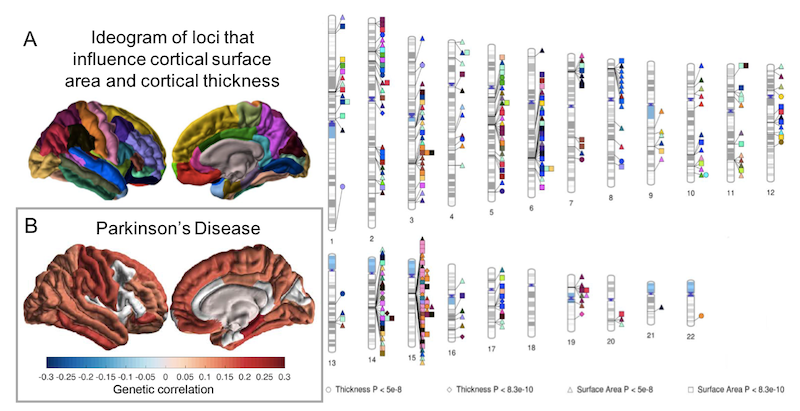
- Max A. Laansma, Joanna K. Bright, Sarah Al-Bachari, Tim J. Anderson, Tyler Ard, Francesca Assogna, Katherine A. Baquero, Henk W. Berendse, Jamie Blair, Fernando Cendes, John C. Dalrymple-Alford, Rob M. A. de Bie, Ines Debove, Michiel F. Dirkx, Jason Druzgal, Hedley C. A. Emsley, Gäetan Garraux, Rachel P. Guimarães, Boris A. Gutman, Rick C. Helmich, Johannes C. Klein, Clare E. Mackay, Corey T. McMillan, Tracy R. Melzer, Laura M. Parkes, Fabrizio Piras, Toni L. Pitcher, Kathleen L. Poston, Mario Rango, Letícia F. Ribeiro, Cristiane S. Rocha, Christian Rummel, Lucas S. R. Santos, Reinhold Schmidt, Petra Schwingenschuh, Gianfranco Spalletta, Letizia Squarcina, Odile A. van den Heuvel, Chris Vriend, Jiun-Jie Wang, Daniel Weintraub, Roland Wiest, Clarissa L. Yasuda, Neda Jahanshad, Paul M. Thompson, Ysbrand D. van der Werf. An International Multicenter Analysis of Brain Structure across Clinical Stages of Parkinson’s Disease: The ENIGMA-Parkinson’s Study. Preprint on medRxiv 2020.2020.04.28.20072710. [link]
- Grasby KL, Jahanshad N, Painter JN, Colodro-Conde L, Bralten J, Hibar DP, Lind PA, Pizzagalli F, Ching CRK, McMahon MAB, Shatokhina N, Zsembik LCP, Thomopoulos SI, Zhu AH, Strike LT, Agartz I, Alhusaini S, Almeida MAA, Alnæs D, Amlien IK, Andersson M, Ard T, Armstrong NJ, Ashley-Koch A, Atkins JR, Bernard M, Brouwer RM, Buimer EEL, Bülow R, Bürger C, Cannon DM, Chakravarty M, Chen Q, Cheung JW, Couvy-Duchesne B, Dale AM, Dalvie S, de Araujo TK, de Zubicaray GI, de Zwarte SMC, den Braber A, Doan NT, Dohm K, Ehrlich S, Engelbrecht HR, Erk S, Fan CC, Fedko IO, Foley SF, Ford JM, Fukunaga M, Garrett ME, Ge T, Giddaluru S, Goldman AL, Green MJ, Groenewold NA, Grotegerd D, Gurholt TP, Gutman BA, Hansell NK, Harris MA, Harrison MB, Haswell CC, Hauser M, Herms S, Heslenfeld DJ, Ho NF, Hoehn D, Hoffmann P, Holleran L, Hoogman M, Hottenga JJ, Ikeda M, Janowitz D, Jansen IE, Jia T, Jockwitz C, Kanai R, Karama S, Kasperaviciute D, Kaufmann T, Kelly S, Kikuchi M, Klein M, Knapp M, Knodt AR, Krämer B, Lam M, Lancaster TM, Lee PH, Lett TA, Lewis LB, Lopes-Cendes I, Luciano M, Macciardi F, Marquand AF, Mathias SR, Melzer TR, Milaneschi Y, Mirza-Schreiber N, Moreira JCV, Mühleisen TW, Müller-Myhsok B, Najt P, Nakahara S, Nho K, Olde Loohuis LM, Orfanos DP, Pearson JF, Pitcher TL, Pütz B, Quidé Y, Ragothaman A, Rashid FM, Reay WR, Redlich R, Reinbold CS, Repple J, Richard G, Riedel BC, Risacher SL, Rocha CS, Mota NR, Salminen L, Saremi A, Saykin AJ, Schlag F, Schmaal L, Schofield PR, Secolin R, Shapland CY, Shen L, Shin J, Shumskaya E, Sønderby IE, Sprooten E, Tansey KE, Teumer A, Thalamuthu A, Tordesillas-Gutiérrez D, Turner JA, Uhlmann A, Vallerga CL, van der Meer D, van Donkelaar MMJ, van Eijk L, van Erp TGM, van Haren NEM, van Rooij D, van Tol MJ, Veldink JH, Verhoef E, Walton E, Wang M, Wang Y, Wardlaw JM, Wen W, Westlye LT, Whelan CD, Witt SH, Wittfeld K, Wolf C, Wolfers T, Wu JQ, Yasuda CL, Zaremba D, Zhang Z, Zwiers MP, Artiges E, Assareh AA, Ayesa-Arriola R, Belger A, Brandt CL, Brown GG, Cichon S, Curran JE, Davies GE, Degenhardt F, Dennis MF, Dietsche B, Djurovic S, Doherty CP, Espiritu R, Garijo D, Gil Y, Gowland PA, Green RC, Häusler AN, Heindel W, Ho BC, Hoffmann WU, Holsboer F, Homuth G, Hosten N, Jack CR Jr, Jang M, Jansen A, Kimbrel NA, Kolskår K, Koops S, Krug A, Lim KO, Luykx JJ, Mathalon DH, Mather KA, Mattay VS, Matthews S, Mayoral Van Son J, McEwen SC, Melle I, Morris DW, Mueller BA, Nauck M, Nordvik JE, Nöthen MM, O'Leary DS, Opel N, Martinot MP, Pike GB, Preda A, Quinlan EB, Rasser PE, Ratnakar V, Reppermund S, Steen VM, Tooney PA, Torres FR, Veltman DJ, Voyvodic JT, Whelan R, White T, Yamamori H, Adams HHH, Bis JC, Debette S, Decarli C, Fornage M, Gudnason V, Hofer E, Ikram MA, Launer L, Longstreth WT, Lopez OL, Mazoyer B, Mosley TH, Roshchupkin GV, Satizabal CL, Schmidt R, Seshadri S, Yang Q; Alzheimer’s Disease Neuroimaging Initiative; CHARGE Consortium; EPIGEN Consortium; IMAGEN Consortium; SYS Consortium; Parkinson’s Progression Markers Initiative, Alvim MKM, Ames D, Anderson TJ, Andreassen OA, Arias-Vasquez A, Bastin ME, Baune BT, Beckham JC, Blangero J, Boomsma DI, Brodaty H, Brunner HG, Buckner RL, Buitelaar JK, Bustillo JR, Cahn W, Cairns MJ, Calhoun V, Carr VJ, Caseras X, Caspers S, Cavalleri GL, Cendes F, Corvin A, Crespo-Facorro B, Dalrymple-Alford JC, Dannlowski U, de Geus EJC, Deary IJ, Delanty N, Depondt C, Desrivières S, Donohoe G, Espeseth T, Fernández G, Fisher SE, Flor H, Forstner AJ, Francks C, Franke B, Glahn DC, Gollub RL, Grabe HJ, Gruber O, Håberg AK, Hariri AR, Hartman CA, Hashimoto R, Heinz A, Henskens FA, Hillegers MHJ, Hoekstra PJ, Holmes AJ, Hong LE, Hopkins WD, Hulshoff Pol HE, Jernigan TL, Jönsson EG, Kahn RS, Kennedy MA, Kircher TTJ, Kochunov P, Kwok JBJ, Le Hellard S, Loughland CM, Martin NG, Martinot JL, McDonald C, McMahon KL, Meyer-Lindenberg A, Michie PT, Morey RA, Mowry B, Nyberg L, Oosterlaan J, Ophoff RA, Pantelis C, Paus T, Pausova Z, Penninx BWJH, Polderman TJC, Posthuma D, Rietschel M, Roffman JL, Rowland LM, Sachdev PS, Sämann PG, Schall U, Schumann G, Scott RJ, Sim K, Sisodiya SM, Smoller JW, Sommer IE, St Pourcain B, Stein DJ, Toga AW, Trollor JN, Van der Wee NJA, van 't Ent D, Völzke H, Walter H, Weber B, Weinberger DR, Wright MJ, Zhou J, Stein JL, Thompson PM, Medland SE; Enhancing NeuroImaging Genetics through Meta-Analysis Consortium (ENIGMA)—Genetics working group. The genetic architecture of the human cerebral cortex. Science. 2020 Mar 20;367(6484):eaay6690. doi: 10.1126/science.aay6690. PMID: 32193296; PMCID: PMC7295264. [link]
ENIGMA World Aging Center
Abnormal brain aging, characterized by neurodegeneration and development of neurocognitive disabilities, is a global health concern, as the population over age 65 increases. In preliminary work supported by an NIA-funded R56 grant, we computed nomograms (percentile charts) of hippocampal volume, discovered 2 epigenetic loci affecting hippocampal volumes, and identified over 15 genetic loci affecting rates of brain tissue loss with age, demonstrating feasibility for a worldwide longitudinal analysis of MRI data in >10,000 people. Building on these data, we will launch the ENIGMA World Aging Center, a global study of brain aging that leverages our vast and highly productive ENIGMA consortium. ENIGMA’s World Aging Center is a concerted global effort to pool all available data, methods, expertise and capital infrastructure to discover genetic and epigenetic variants that affect brain aging. We focus on biological predictors of aging as they are more mechanistically interpretable. Our long-term goal is to identify personalized biological predictors of brain structural and functional decline, determine who they affect and when, and assess how they generalize globally. See more here: http://ewac.ini.usc.edu/
Further Reading
- Brouwer RM, Klein M, Grasby KL, Schnack HG, Jahanshad N, Teeuw J, [members of the ENIGMA-Plasticity Working Group], Medland SE, Franke B, Thompson PM, Hulshoff Pol HE. Dynamics of Brain Structure and its Genetic Architecture over the Lifespan. bioRxiv. 2020 Jan 1;2020.04.24.031138. [link]
- Lawrence KE, Nabulsi L, Santhalingam V, Abaryan Z, Villalon-Reina JE, Nir TM, Ba Gari I, Zhu AH, Haddad E, Muir AM, Jahanshad N, Thompson PM. Advanced diffusion-weighted MRI metrics detect sex differences in aging among 15,000 adults in the UK Biobank. bioRxiv. 2020 Jan 1;2020.09.18.304345. [link]
- Nabulsi L, Lawrence KE, Santhalingam V, Abaryan Z, Boyle CP, Villalon-Reina JE, Nir TM, Ba Gari I, Zhu AH, Haddad E, Muir AM, Jahanshad N, Thompson PM. (2020). Exogenous sex hormone effects on brain microstructure in women: a diffusion MRI study in the UK Biobank. BioRxiv. 2020.09.18.304154. doi:10.1101/2020.09.18.304154 [link]
Psychiatric Disorders & Global Mental Health
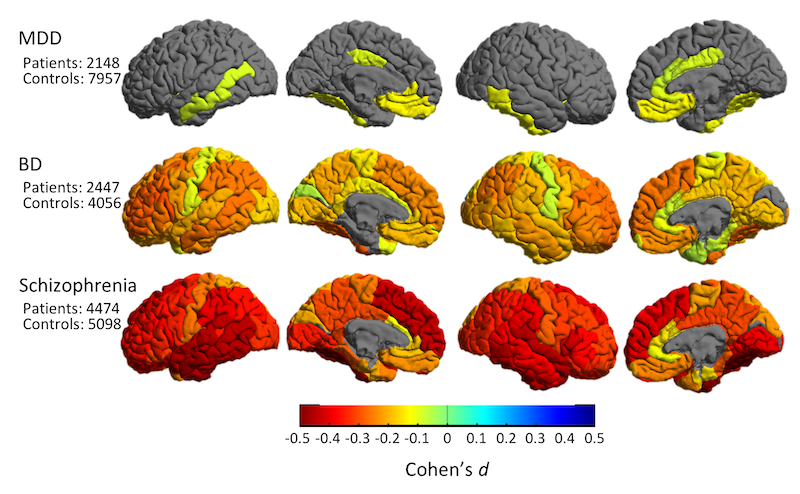
The ENIGMA-Sex Differences is the first, large scale, concerted effort to study sex as a biological variable in human neuroscience through a worldwide brain initiative. Our proposal is a response to the NIH mandate to address the major gaps in knowledge about sex differences in common and debilitating brain disorders. This is a critical grant initiative, as women and men differ in neurobiology, phenotypic presentation of aging and clinical syndromes, and prevalence of psychiatric disease. Specifically, male and female brains differ in size, connectivity patterns and aging trends and also in the prevalence of major depression, anxiety disorders, substance abuse disorders, alcoholism, psychosis, post-traumatic stress, and neurodegenerative diseases such as Alzheimer’s disease. There are significant sex differences in the disorders’ mean age of onset, treatment response, symptom severity and disease trajectory. However, there is limited understanding of the mechanisms and explanatory factors that contribute to these differences. Even the largest brain imaging studies are underpowered to determine if the impact of these diseases in the brain differs by sex. This lack of knowledge - even of basic trajectories of brain disease in men versus women - stalls efforts to identify the primary risk factors in the genome and environment that account for sex differences in disease. Recognizing this major knowledge gap, NIH has repeatedly asked investigators to study sex differences in disease risks, prevalence, trajectories and outcomes.
Further Reading
- Ching CRK, Hibar DP, Gurholt TP, Nunes A, Thomopoulos SI, Abé C, Agartz I, Brouwer RM, Cannon DM, de Zwarte SMC, Eyler LT, Favre P, Hajek T, Haukvik UK, Houenou J, Landén M, Lett TA, McDonald C, Nabulsi L, Patel Y, Pauling ME, Paus T, Radua J, Soeiro-de-Souza MG, Tronchin G, van Haren NEM, Vieta E, Walter H, Zeng LL, Alda M, Almeida J, Alnaes D, Alonso-Lana S, Altimus C, Bauer M, Baune BT, Bearden CE, Bellani M, Benedetti F, Berk M, Bilderbeck AC, Blumberg HP, Bøen E, Bollettini I, Del Mar Bonnin C, Brambilla P, Canales-Rodríguez EJ, Caseras X, Dandash O, Dannlowski U, Delvecchio G, Díaz-Zuluaga AM, Dima D, Duchesnay É, Elvsåshagen T, Fears SC, Frangou S, Fullerton JM, Glahn DC, Goikolea JM, Green MJ, Grotegerd D, Gruber O, Haarman BCM, Henry C, Howells FM, Ives-Deliperi V, Jansen A, Kircher TTJ, Knöchel C, Kramer B, Lafer B, López-Jaramillo C, Machado-Vieira R, MacIntosh BJ, Melloni EMT, Mitchell PB, Nenadic I, Nery F, Nugent AC, Oertel V, Ophoff RA, Ota M, Overs BJ, Pham DL, Phillips ML, Pineda-Zapata JA, Poletti S, Polosan M, Pomarol-Clotet E, Pouchon A, Quidé Y, Rive MM, Roberts G, Ruhe HG, Salvador R, Sarró S, Satterthwaite TD, Schene AH, Sim K, Soares JC, Stäblein M, Stein DJ, Tamnes CK, Thomaidis GV, Upegui CV, Veltman DJ, Wessa M, Westlye LT, Whalley HC, Wolf DH, Wu MJ, Yatham LN, Zarate CA, Thompson PM, Andreassen OA; ENIGMA Bipolar Disorder Working Group. What we learn about bipolar disorder from large-scale neuroimaging: Findings and future directions from the ENIGMA Bipolar Disorder Working Group. Hum Brain Mapp. 2020 Jul 29. doi:10.1002/hbm.25098. Epub ahead of print. PMID: 32725849. [link]
- Salminen L, Tubi M, Bright J, Thompson P. Sex disparities in psychiatric and neurodegenerative disorders: Insights from large-scale neuroimaging [Internet]. PsyArXiv. 2020. doi:10.31234/osf.io/m59dg [link]
India ENIGMA Initiative
Our proposal launches a globally coordinated study of brain aging and Alzheimer’s disease (AD). This India ENIGMA Initiative for Global Aging & Mental Health offers an innovative, powerful and cost-effective approach to determine common and unique factors that affect brain aging in both India and the U.S. The worldwide burden of AD is severe and universal; we are in dire need of internationally coordinated efforts to better understand variables that affect brain aging and progression to dementia in diverse settings. The incidence of AD is significantly lower in rural India than in semi-urban regions and both are lower than in the U.S. (Chandra 2001, Mathuranath 2012); this offers a unique opportunity to study factors that may promote resilience to brain aging and dementia. Even so, neuroimaging and genetic studies of brain aging and AD in India are limited - largely due to the challenging research infrastructure of India.
As a low and middle-income country (LMIC), India lacks sufficient resources to train highly motivated medical research personnel to change the current landscape of clinical research (Patel 2010). More than 21,000 postgraduates are in medical training positions in India, yet few will be offered sufficient training to conduct novel research and publish in peer-reviewed medical journals. Fortunately, pioneering neuroscientists at the National Institute of Mental Health & Neuro Sciences (NIMHANS) in Bangalore, India, have begun to develop large-scale Indian biobanks with the potential to uncover pivotal insights about global health threats, such as AD, and factors that delay its clinical progression.
To address this gap in knowledge, we launch a new initiative, led by highly productive scientists at NIMHANS and the University of Southern California (USC) - two leading institutions in global mental health. Our new India ENIGMA Initiative builds on our thriving ENIGMA Consortium (enigma.usc.edu). Given NIH’s call for better reproducibility in science (Ioannidis 2014), our initiative aims to identify universal and specific biomarkers of brain aging and risk for AD. We will leverage ENIGMA’s successful capacity building strategies to train emerging and established scientists in India to analyze their data with high quality control and precision, in harmonized efforts that support global comparisons. This collaborative partnership between USC and NIMHANS will enable current and future science initiatives, and equip the NIMHANS team with the necessary tools to train new scientists and independently conduct high impact research bridging efforts into numerous international partnerships.
Currently, we are investigating differential trajectories of brain atrophy between men and women across adulthood (ages 45 - 80) in a subsample of individuals with self-reported Indian ancestry in the UK Biobank. We have created normative centile curves (also known as nomograms) for subcortical structures, namely the accumbens, amygdala, caudate, hippocampus, thalamus, pallidum, putamen, and ventricles. These nomograms can provide references for healthy aging of subcortical structures in men and women.
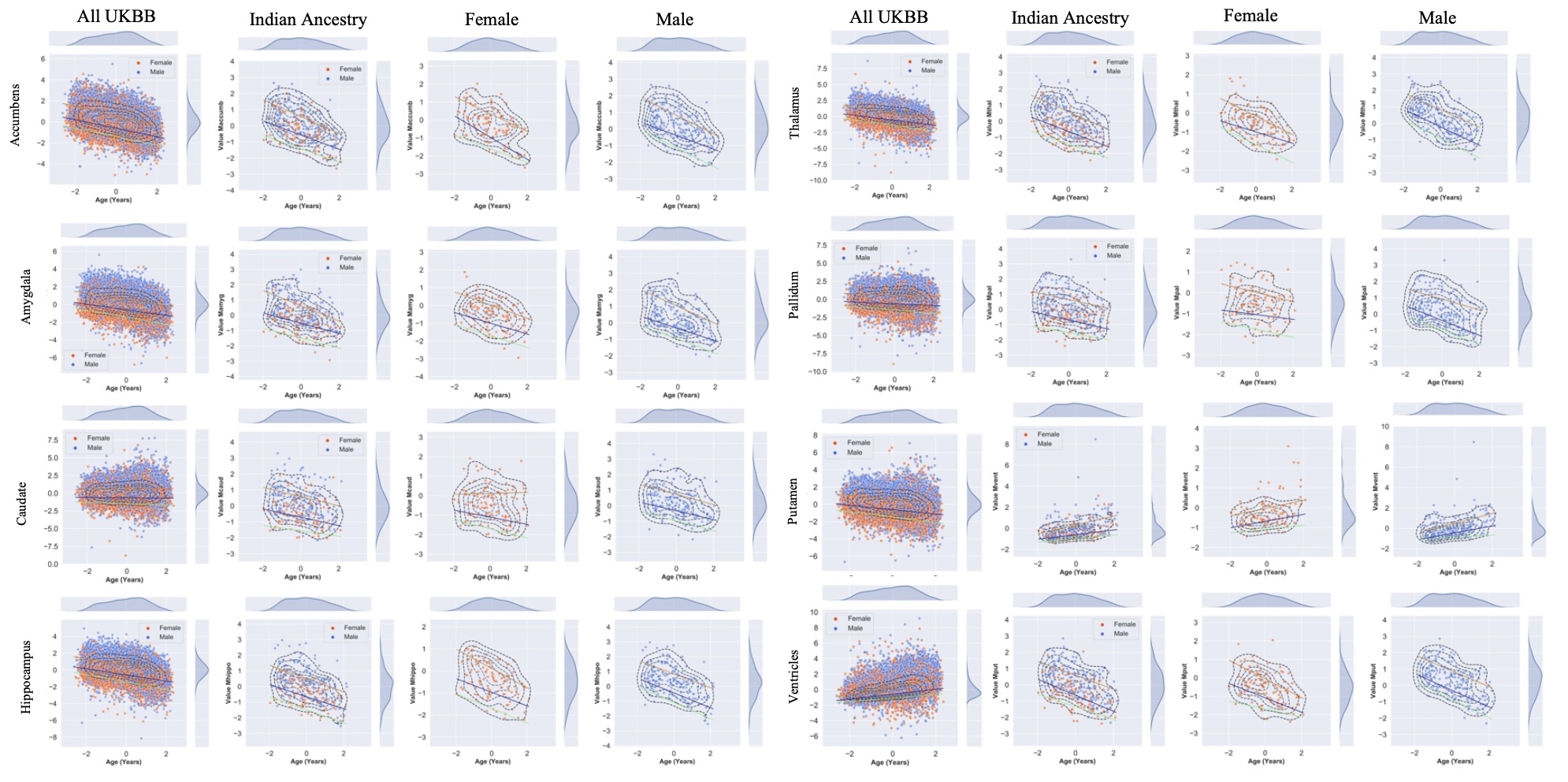
Additionally, we created nomograms for various measures of white matter integrity in a subsample of individuals with self-reported Indian ancestry, again to provide a normative reference of aging in dMRI measures.
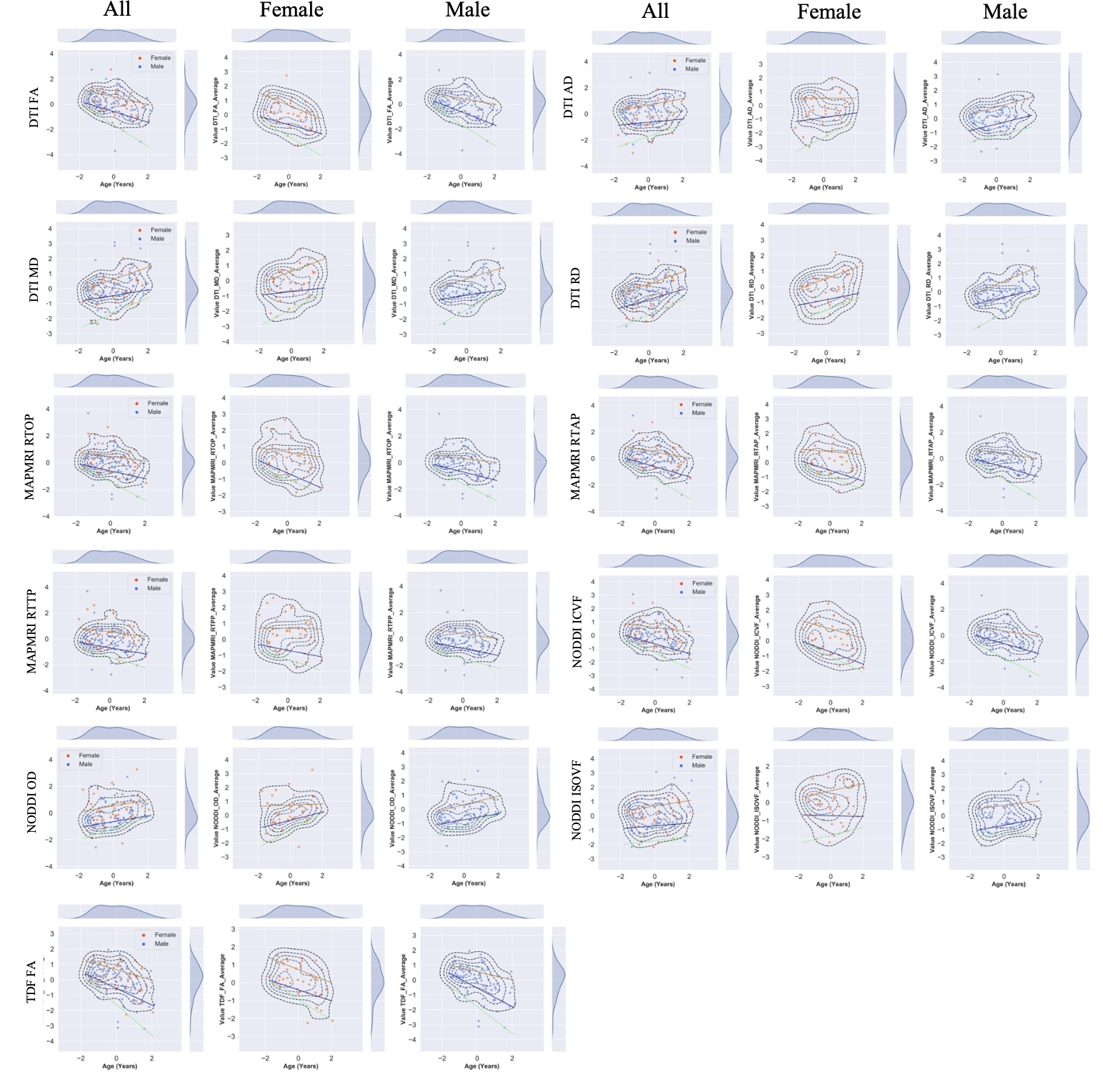 Normative centile curves across whole-brain metrics diffusivity metrics. Colored lines indicate 5th (black), 25th (blue), 50th (red), 75th (green), 95th (purple) centiles. Sampling density or distribution curves for age and each diffusion metric are shown at the top and right of each graph. X-axis representing age, standardized and mean centered at 0. Centile values were stratified by sex. Curves show age-dependent trajectories for all metrics.FA=fractional anisotropy; MD=mean diffusivity; AD=axial diffusivity; RD=radial diffusivity; OD=orientation dispersion; ICVF=intra-cellular volume fraction; ISOVF=isotropic volume fraction; RTOP=return-to-origin probability; RTPP=return-to-plane probability; RTAP=return-to-axis probability.
Normative centile curves across whole-brain metrics diffusivity metrics. Colored lines indicate 5th (black), 25th (blue), 50th (red), 75th (green), 95th (purple) centiles. Sampling density or distribution curves for age and each diffusion metric are shown at the top and right of each graph. X-axis representing age, standardized and mean centered at 0. Centile values were stratified by sex. Curves show age-dependent trajectories for all metrics.FA=fractional anisotropy; MD=mean diffusivity; AD=axial diffusivity; RD=radial diffusivity; OD=orientation dispersion; ICVF=intra-cellular volume fraction; ISOVF=isotropic volume fraction; RTOP=return-to-origin probability; RTPP=return-to-plane probability; RTAP=return-to-axis probability.
 Normative centile curves across corpus callosum diffusivity metrics. Colored lines indicate 5th (black), 25th (blue), 50th (red), 75th (green), 95th (purple) centiles. Sampling density or distribution curves for age and each diffusion metric are shown at the top and right of each graph. X-axis representing age, standardized and mean centered at 0. Centile values were stratified by sex. Curves show age-dependent trajectories for all metrics. CC=corpus callosum.
Normative centile curves across corpus callosum diffusivity metrics. Colored lines indicate 5th (black), 25th (blue), 50th (red), 75th (green), 95th (purple) centiles. Sampling density or distribution curves for age and each diffusion metric are shown at the top and right of each graph. X-axis representing age, standardized and mean centered at 0. Centile values were stratified by sex. Curves show age-dependent trajectories for all metrics. CC=corpus callosum.