Applied Machine Learning
Learning Anatomic Representations
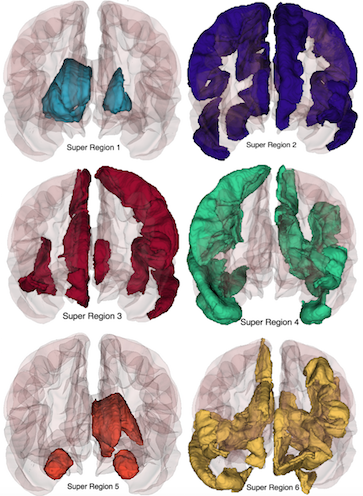
Disease classification tasks based on imaging data have recently become quite popular, due in part to the deluge of structural and diffusion MRI data from clinical studies. However, many of these datasets use standardized anatomic annotation; while this is helpful for sharing results, it may not be an optimal representation with respect to the task at hand, nor to the intrinsic distribution of the data. We currently are exploring alterative, data-driven anatomies, both in supervised contexts (classification, regression, etc.) as well as unsupervised settings.
Our current projects include EPIC, a classification-based optimization of cortical regions, and CorEx (in collaboration with Greg Ver Steeg of the Information Sciences Institute), which is an unsupervised method for finding maximally descriptive hierarchical representations under a mutual information criterion. Other projects include using Stochastic Blockmodels to learn latent community (anatomic) structures in connectivity data.
Further Reading
-
G Prasad, SH Joshi, and PM Thompson. "Optimizing brain connectivity networks for disease classification using EPIC." Proceedings/IEEE International Symposium on Biomedical Imaging: from nano to macro. IEEE International Symposium on Biomedical Imaging.
Vol. 2014. NIH Public Access, 2014. [link]
-
M Daianu, G Ver Steeg, A Mezher, N Jahanshad, TM Nir, X Yan, and PM Thompson "Information-theoretic clustering of neuroimaging metrics related to cognitive decline in the elderly." International MICCAI Workshop on Medical Computer Vision. Springer International
Publishing, 2015. [link]
- D Moyer, B Gutman, G Prasad, J Faskowitz, G Ver Steeg, and PM Thompson "Blockmodels for connectome analysis." 11th International Symposium on Medical Information Processing and Analysis (SIPAIM 2015). International Society for Optics and Photonics, 2015. [ link]
Automated White Matter/Fiber Tract Segmentation
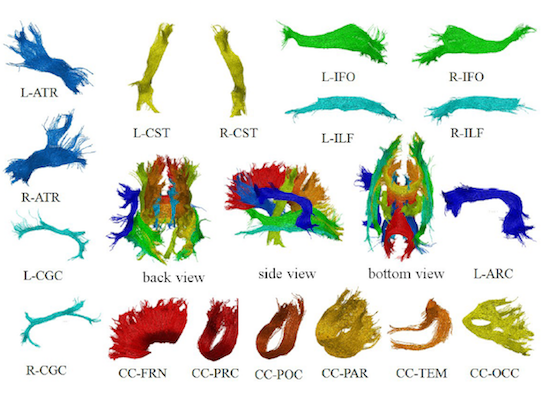
Using Diffusion weighted MRI, we can can reconstruct white matter pathways in the brain; this process is referred to as tractography. The study and analysis of these recovered tracts allows researchers to better understand white matter pathologies under a variety of effects, ranging from traumatic brain injury (and the recovery thereof) to HIV treamtents.
One undesirable and as-of-yet unavoidable characteristic of tractography is the presence of noisy or false-positive fibers. The presence of these noisy fibers can confound clinical studies of the aforementioned pathologies, and make the identification of known WM matter fiber bundles difficult. A clustering algorithm developed by our group can help mediate these noisy, outlier fibers so that researchers can examine WM fiber bundles specific to their interest (Jin et al., 2014).
Automated Multi-Atlas Tract Extraction, or autoMATE for short, allows us to visualize and quantify the full 3D profile of white fiber bundles. It uses a human-in-the-loop strategy, augmenting expert ability to reduce actual human workload while maintaining quality. After a whole-brain tractography is generated, 5 manually reconstructed atlases are created by an anatomical expert. These atlases have specific parcellations of known white matter pathways of the brain. Next, a multi-level fiber clustering scheme is applied to generate bundles from the new subjects.
Further Reading:
- Thomason, M.E., Thompson, P.M., 2011. Diffusion imaging, white matter, and psychopathology. Annu. Rev. Clin. Psychol. 7, 63–85. [link]
- Jin, Y., Shi, Y., Zhan, L., Gutman, B. A., de Zubicaray, G. I., McMahon, K. L., Wright, M. J., Toga, A. W., & Thompson, P. M. (2014). Automatic clustering of white matter fibers in brain diffusion MRI with an application
to genetics. NeuroImage, 100, 75–90. [link]
- Daianu, M., Jahanshad, N., Nir, T.M., Toga, A.W., Jack Jr., C.R., Weiner, M.W., Thompson, P.M., 2013. Alzheimer's disease neuroimaging initiative. Breakdown of brain connectivity between normal aging and Alzheimer's disease: a structural k-core network analysis. Brain Connect. 3 (4), 407–422. [link]
ENIGMA
Founded in 2009, the ENIGMA (Enhancing Neuro Imaging Genetics through Meta-Analysis) Consortium is a collaboration of 500+ multi-disciplinary scientists from 37 countries working together to discover factors that help or harm the human brain. ENIGMA is comprised of about 30 working groups that study major brain diseases, healthy development, and novel methods in several neuroimaging and genetic modalities. Since the beginning, the worldwide network of the ENIGMA Consortium has grown exponentially. Pooling brain scans from now over 50,000 people, ENIGMA analyses and tools have also progressed the work beyond sheer numbers, which yield the largest-ever studies of their kind. The NIH awarded ENIGMA $11 million in 2014, making it a Big Data Center of Excellence aiming to "crack the brain’s genetic code.” ENIGMA does this by:
(1) creating an international network of multidisciplinary scientists
(2) ensuring replication via member collaborations
(3) facilitating shared information, data, and methods
(4) emphasizing scientific training and exploring emerging directions
Please see the official ENIGMA website here.
Interactive Tableau visualization of Disease Working Groups in ENIGMA, plotted by longitude and latitude with color by Working Group. Tooltips include PI and site details.
Interactive Tableau visualization of Disease Working Groups in ENIGMA, showing the effects of genes on brain changes across life. The map plots longitude and latitude, uses color to indicate working groups, and includes tooltips for site and principal investigator. Press Tab to move to the next section.